from Gibbs Equation,
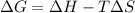
reaction is spontaneous if $\Delta G$ is negative.
so, first option is not valid at high temperature, ($-h+ts$)
second, is always a spontaneous reaction, ($-h-ts$)
third, is never spontaneous ($+h+ts$)
4th is similar to second, spontaneous at higher temperatures ($+h-ts$)