☃️ Chemical formulae ➝
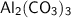
How to find?
For solving this question, We need to know how to find moles of solution or any substance if a certain weight is given.
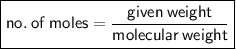
Solution:
❍ Molecular weight of
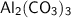
= 2 × 27 + 3 × 12 + 9 × 16
= 54 + 36 + 144
= 234
❍ Given weight: 10 g
Then, no. of moles,
⇛ No. of moles = 10 / 234
⇛ No. of moles = 0.0427 moles
⚘ No. of moles of Aluminium carbonate in the given weight = 0.0427 moles.
━━━━━━━━━━━━━━━━━━━━