☃️ Chemical formulae ➝
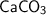
How to find?
For solving this question, We need to know how to find moles of solution or any substance if a certain weight is given.
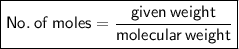
Solution:
Atomic weight of elements:
Ca = 40
C = 12
O = 16
❍ Molecular weight of
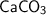
= 40 + 12 + 3 × 16
= 52 + 48
= 100 g/mol
❍ Given weight: 10 g
Then, no. of moles,
⇛ No. of moles = 10 g / 100 g mol‐¹
⇛ No. of moles = 0.1 moles
☄ No. of moles of Calcium carbonate in that substance = 0.1 moles
━━━━━━━━━━━━━━━━━━━━