Answer:
The answer is 0.089 mol
Step-by-step explanation:
The number of moles of a substance can be found by
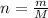
where
n is the number of moles
M is the molar mass
m is the mass of the substance
To find the number of moles we must first find the molar mass
Chemical formula for calcium oxide is
CaO
So the molar mass of CaO is
M( CaO) = 40 + 16 = 56 g/mol
Mass of CaO = 5.0g
Substitute the given values into the formula
The number of moles in 5.0g of calcium oxide is
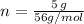
We have the final answer as
n = 0.089 mol
Hope this helps you