Answer:
The answer is 0.08mol
Step-by-step explanation:
To find the number of moles in calcium phosphate we use the formula
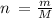
where
n is the number of moles
M is the molar mass
m is the mass of the substance
To find the number of moles we must first find the molar mass
Chemical formula for calcium phosphate is
Ca3( PO4)2
Ca = 40 , P = 31 , O = 16
So the Molar mass of calcium phosphate is
M( Ca3( PO4)2 ) = (20×3) + 2( 31 + (16×4)
= 60 + 2( 31 + 64)
= 60 + 2(95)
= 60 + 190
Molar mass of calcium phosphate =
250g/mol
Mass = 20 g
So we have
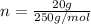
We have the final answer as
n = 0.08 mol
Hope this helps you