Answer:
33 mL
Step-by-step explanation:
It is important to recognize that the total amount of pure syrup remains constant:
Total syrup in the stock solution = total syrup in dilute solution
Thus, we can use the dilution formula
V₁c₁ = V₂ c₂
Data:
V₁ = ?; c₁ = 80 %
V₂ = 47 mL; c₂ = 24 %
Calculations:
1. The volume of stock syrup
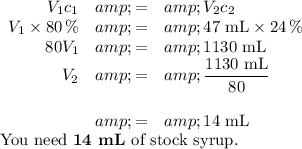
2. The volume of soda solution
Total volume of dilute solution = 47 mL
The volume of stock syrup = 14
Volume of soda solution = 33 mL
You need 33 mL of soda solution.