Answer:
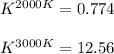
Step-by-step explanation:
Hello,
In this case, considering the reaction, we can compute the Gibbs free energy of reaction at each temperature, taking into account that the Gibbs free energy for the diatomic element is 0 kJ/mol:
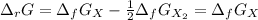
Thus, at 2000 K:
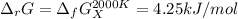
And at 3000 K:
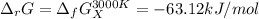
Next, since the relationship between the equilibrium constant and the Gibbs free energy of reaction is:
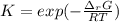
Thus, at each temperature we obtain:
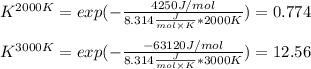
In such a way, we can also conclude that at 2000 K reaction is unfavorable (K<1) and at 3000 K reaction is favorable (K>1).
Best regards.