Answer:
![[F^-]_(max)=4x10{-3}(molF^-)/(L)](https://img.qammunity.org/2021/formulas/chemistry/college/gws3ttoiyf7o0ne3z334ae6rjsv7nze4z3.png)
Step-by-step explanation:
Hello,
In this case, for the described situation, we infer that calcium reacts with fluoride ions to yield insoluble calcium fluoride as shown below:
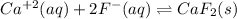
Which is typically an equilibrium reaction, since calcium fluoride is able to come back to the ions. In such a way, since the maximum amount is computed via stoichiometry, we can see a 1:2 mole ratio between the ions, therefore, the required maximum amount of fluoride ions in the "hard" water (assuming no other ions) turns out:
![[F^-]_(max)=2.0x10^(-3)(molCa^(2+))/(L)*(2molF^-)/(1molCa^(2+)) \\](https://img.qammunity.org/2021/formulas/chemistry/college/fh8y97uk052rc2xp85oy24canuhs2agw3d.png)
![[F^-]_(max)=4x10{-3}(molF^-)/(L)](https://img.qammunity.org/2021/formulas/chemistry/college/gws3ttoiyf7o0ne3z334ae6rjsv7nze4z3.png)
Best regards.