Answer:
 and
and
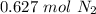
Step-by-step explanation:
Our goal for this question is the calculation of the number of moles of the molecules produced by the reaction of hydrazine (
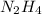 ) and oxygen (
) and oxygen (
 ). So, we can start with the reaction between these compounds:
). So, we can start with the reaction between these compounds:

Now we can balance the reaction:

In the problem, we have the values for both reagents. Therefore we have to calculate the limiting reagent. Our first step, is to calculate the moles of each compound using the molar masses values (32.04 g/mol for
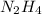 and 31.99 g/mol for
and 31.99 g/mol for
 ):
):
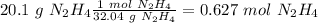
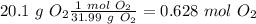
In the balanced reaction we have 1 mol for each reagent (the numbers in front of
 and
and
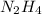 are 1). Therefore the smallest value would be the limiting reagent, in this case, the limiting reagent is
are 1). Therefore the smallest value would be the limiting reagent, in this case, the limiting reagent is
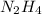 .
.
With this in mind, we can calculate the number of moles for each product. In the case of
 we have a 1:1 molar ratio (1 mol of
we have a 1:1 molar ratio (1 mol of
 is produced by 1 mol of
is produced by 1 mol of
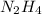 ), so:
), so:
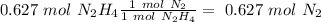
We can follow the same logic for the other compound. In the case of
 we have a 1:2 molar ratio (2 mol of
we have a 1:2 molar ratio (2 mol of
 is produced by 1 mol of
is produced by 1 mol of
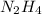 ), so:
), so:
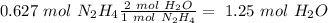
I hope it helps!