Answer:
The volume will decrease by a factor of 10/51.
Step-by-step explanation:
Hello,
In this case, since both moles and temperature remain constant, we can use the Boyle's law that relates the volume and pressure as an inversely proportional relationship:

Thus, since the pressure increases by a factor of 5.1 (statement), we have:
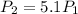
Thus, the final volume is:
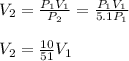
It means that the volume will decrease by a factor of 10/51.
Regards.