Answer:
The energy of one of its photons is 1.391 x 10⁻¹⁸ J
Step-by-step explanation:
Given;
wavelength of the UVC light, λ = 142.9 nm = 142.9 x 10⁻⁹ m
The energy of one photon of the UVC light is given by;
E = hf
where;
h is Planck's constant = 6.626 x 10⁻³⁴ J/s
f is frequency of the light
f = c / λ
where;
c is speed of light = 3 x 10⁸ m/s
λ is wavelength
substitute in the value of f into the main equation;
E = hf
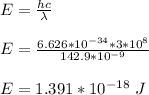
Therefore, the energy of one of its photons is 1.391 x 10⁻¹⁸ J