Answer: Reaction (1) , (3) and (4) are accompanied by an increase in entropy.
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
(1)
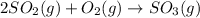
3 moles of reactant are changing to 1 mole of product , thus the randomness is increasing. Thus the entropy also increases.
2)
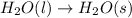
1 mole of Liquid reactant is changing to 1 mole of solid product , thus the randomness is decreasing. Thus the entropy also decreases.
3)
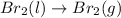
1 mole of Liquid reactant is changing to 1 mole of gaseous product , thus the randomness is increasing. Thus the entropy also increases.
4)
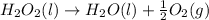
1 mole of Liquid reactant is changing to half mole of gaseous product and 1 mole of liquid product, thus the randomness is increasing. Thus the entropy also increases.