Answer:
The amount of heat that is absorbed when 3.11 g of water boils at atmospheric pressure is 7.026 kJ.
Step-by-step explanation:
A molar heat of vaporization of 40.66 kJ / mol means that 40.66 kJ of heat needs to be supplied to boil 1 mol of water at its normal boiling point.
To know the amount of heat that is absorbed when 3.11 g of water boils at atmospheric pressure, the number of moles represented by 3.11 g of water is necessary. Being:
the molar mass of water is:
H₂O= 2* 1 g/mole + 16 g/mole= 18 g/mole
So: if 18 grams of water are contained in 1 mole, 3.11 grams of water in how many moles are present?
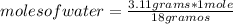
moles of water= 0.1728
Finally, the following rule of three can be applied: if to boil 1 mole of water at its boiling point it is necessary to supply 40.66 kJ of heat, to boil 0.1728 moles of water, how much heat is necessary to supply?
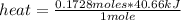
heat= 7.026 kJ
The amount of heat that is absorbed when 3.11 g of water boils at atmospheric pressure is 7.026 kJ.