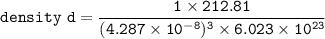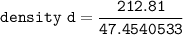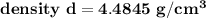Answer:
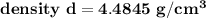
Step-by-step explanation:
Let recall the crystal structure of CsBr obtains a BCC structure. In a BCC structure, there exist only two atom per cell.
The density d of CsBr in g/cm³ can be calculated by using the formula:
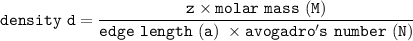
where;
z = 1 mole of CsBr
edge length = 428.7 pm = (4.287 × 10⁻⁸)³ cm
molar mass of CsBr = 212.81 g/mol
avogadro's number = 6.023 × 10²³