Answer:
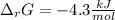
Step-by-step explanation:
Hello,
In this case, for the given dissociation reaction, we can compute the enthalpy of reaction considering the enthalpy of formation of each involved species (products minus reactants):
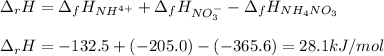
Next, the entropy of reaction considering the standard entropy for each involved species (products minus reactants):
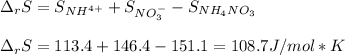
Next, since the Gibbs free energy of reaction is computed in terms of both the enthalpy and entropy of reaction at the given temperature (298.15 K), we finally obtain (two significant figures):
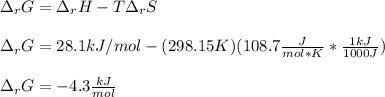
Best regards.