Answer:
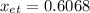
Step-by-step explanation:
Hello,
In this case, since the mole fraction of a compound, in this case ethanol, in a binary mixture, in this constituted by both water and ethanol, is mathematically defined as follows:
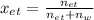
Whereas
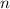 accounts for the moles in the solution for each species, we must first compute the moles of both ethanol (density: 0.789 g/mL and molar mass: 46.07 g/mol) and water (density: 1g/mL and molar mass: 18.02 g/mol)
accounts for the moles in the solution for each species, we must first compute the moles of both ethanol (density: 0.789 g/mL and molar mass: 46.07 g/mol) and water (density: 1g/mL and molar mass: 18.02 g/mol)
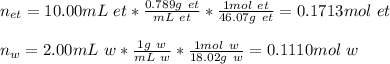
Therefore, the mole fraction turns out:
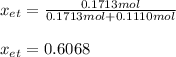
Best regards.