Answer:
The answer is "Option B"
Step-by-step explanation:
when we react with FeCl, iron, and chloride to both the electrolysis. So, iron is charged positive ion, as well as passes to the negative, is cathode electrode. But there is a decrease in the cathode.
The equation can be defined as follows:
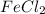

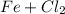
In the above equation, when the iron chloride electrolysis solution, it will give the iron(II) and the chlorine. This process happens when the hydrogen chloride solution at a temperature of about 90 ° C.