Answer: A.
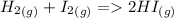 and D.
and D.
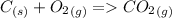
Explanation: The relationship between internal energy change and enthalpy change during a chemical reaction occurs according to the following formula:
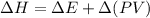
So, for changes in enthalpy and internal energy to be equal volume or pressure has to be constant, i.e., zero.
Change in the number of moles of gas during the reaction can make the difference between
 and
and
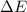 be larger, so for them to be equal and pressure constant, number of moles must be the same in reagents and products.
be larger, so for them to be equal and pressure constant, number of moles must be the same in reagents and products.
Analysing each reaction above:
Reaction A has the same number of moles in reagents and products, so enthalpy change and internal energy change will be equal;
Reactions B and C don't have the same number of moles at both sides, so enthalpy and energy will be different.
Reaction D, although reagent side have 2 compounds, carbon is solid, so reaction have the same number of moles in both sides. Enthalpy and Energy will be equal.