Answer:
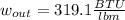
Step-by-step explanation:
Hello,
In this case, for the inlet stream, from the steam table, the specific enthalpy and entropy are:
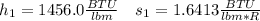
Next, for the liquid-vapor mixture at the outlet stream we need to compute its quality by taking into account that since the turbine is adiabatic, the entropy remains the same:
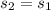
Thus, the liquid and liquid-vapor entropies are included to compute the quality:
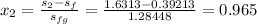
Next, we compute the outlet enthalpy by considering the liquid and liquid-vapor enthalpies:
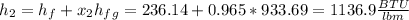
Then, by using the first law of thermodynamics, the maximum specific work is computed via:
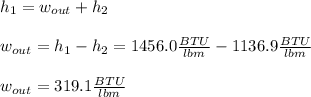
Best regards.