Answer:
The frequency of the photon is 7.41*10¹⁶ Hz
Step-by-step explanation:
Planck states that light is made up of photons, whose energy is directly proportional to the frequency of radiation, according to a constant of proportionality, h, which is called Planck's constant. This is expressed by:
E = h*v
where E is the energy, h the Planck constant (whose value is 6.63*10⁻³⁴ J.s) and v the frequency (Hz or s⁻¹).
So the frequency will be:
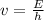
Being E= 4.91*10⁻¹⁷ J and replacing:
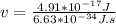
You can get:
v= 7.41*10¹⁶
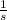 = 7.41*10¹⁶ Hz
= 7.41*10¹⁶ Hz
The frequency of the photon is 7.41*10¹⁶ Hz