Answer:
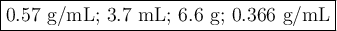
Step-by-step explanation:
1. Density from mass and volume

2. Volume from density and mass
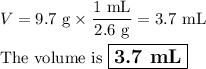
3. Mass from density and volume
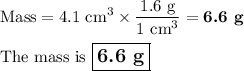
4. Density by displacement
Volume of water + object = 24.6 mL
Volume of water = 12.8 mL
Volume of object = 11.8 mL
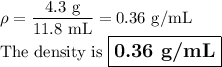
Your drawing showing water displacement using a graduated cylinder should resemble the figure below.