A 1.0-L buffer solution contains 0.100 mol HC2H3O2 and 0.100 mol NaC2H3O2. The value of Ka for HC2H3O2 is 1.8×10−5.
Calculate the pH of the solution, upon addition of 0.035 mol of NaOH to the original buffer.
Answer:
The pH of this solution = 5.06
Step-by-step explanation:
Given that:
number of moles of CH3COOH = 0.100 mol
volume of the buffer solution = 1.0 L
number of moles of NaC2H3O2 = 0.100 mol
The objective is to Calculate the pH of the solution, upon addition of 0.035 mol of NaOH to the original buffer.
we know that concentration in mole = Molarity/volume
Then concentration of [CH3COOH] =
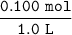 = 0.10 M
= 0.10 M
The chemical equation for this reaction is :
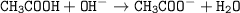
The conjugate base is CH3COO⁻
The concentration of the conjugate base [CH3COO⁻] is =
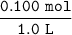
= 0.10 M
where the pka (acid dissociation constant)for CH3COOH = 4.74
If 0.035 mol of NaOH is added to the original buffer, the concentration of NaOH added will be =
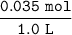 = 0.035 M
= 0.035 M
The ICE Table for the above reaction can be constructed as follows:

Initial 0.10 0.035 0.10 -
Change -0.035 -0.035 + 0.035 -
Equilibrium 0.065 0 0.135 -
By using Henderson-Hasselbalch equation:
The pH of this solution = pKa + log
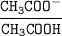
The pH of this solution = 4.74 + log
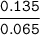
The pH of this solution = 4.74 + log (2.076923077 )
The pH of this solution = 4.74 + 0.3174
The pH of this solution = 5.0574
The pH of this solution = 5.06 to two decimal places