Answer:
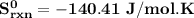
Based upon the stoichiometry of the reaction the sign of Sºrxn should be negative
Step-by-step explanation:
Consider the reaction:
H2CO(g) + O2(g) --------> CO2(g) + H2O(l)
Using standard thermodynamic data;
Based upon the stoichiometry of the reaction the sign of Sºrxn should be _________ . calculate Sºrxn at 25°C. Sºrxn = J/K•mol
At standard thermodynamic data
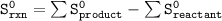
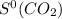 = 213.79 J/mol.K
= 213.79 J/mol.K
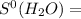 69.95 J/mol.K
69.95 J/mol.K
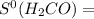 218.95 J/mol.K
218.95 J/mol.K
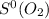 = 205.2 J/mol.K
= 205.2 J/mol.K
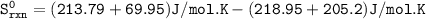
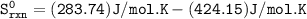
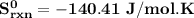
Based upon the stoichiometry of the reaction the sign of Sºrxn should be negative