Answer:
C) 0.027
Step-by-step explanation:
In this case we can start with the reaction between
 and
and
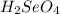 , so:
, so:
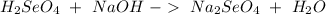
We have an acid (
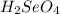 ) and a base (
) and a base (
 ), therefore we will have an acid-base reaction in which a salt is produced (
), therefore we will have an acid-base reaction in which a salt is produced (
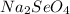 ) and water (
) and water (
 ).
).
Now we can balance the reaction:
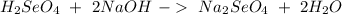
If we have the volume (45 mL= 0.045 L) and the concentration (0.3 M) of the acid we can calculate the moles using the molarity equation:
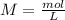
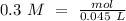
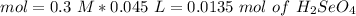
In the balanced reaction, we have a 2:1 molar ratio between the acid and the base (for each mol of
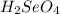 2 moles of
2 moles of
 are consumed), with this in mind we can calculate the moles of NaOH:
are consumed), with this in mind we can calculate the moles of NaOH:
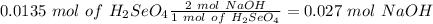
I hope it helps!