Answer:
(a)
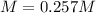
(b)
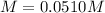
(c)
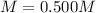
(d)
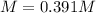
Step-by-step explanation:
Hello,
In this case, since the molarity or molar concentration of a solution is computed by dividing the moles of solute by the volume of solution in liters, we proceed as follows:
(a) The molar mass of sodium chloride is 58.45 g/mol and the volume in liters is 0.100 L, therefore, the molarity is:
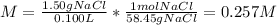
(b) The molar of potassium dichromate is 294.2 g/mol and the volume in liters is 0.100 L, therefore, the molarity is:
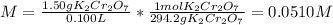
(c) The molar of calcium chloride is 111 g/mol and the volume in liters is 0.125 L, therefore, the molarity is:
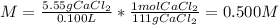
(d) The molar of sodium sulfate is 142 g/mol and the volume in liters is 0.125 L, therefore, the molarity is:
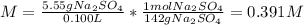
Best regards.