Answer:
The correct option is;
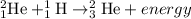
Step-by-step explanation:
The second step of the fusion process is the reaction (combination) where a Deuterium combines with a hydrogen to produce one helium 3, 3He, nucleus and a energy photon as follows;
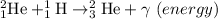
After which the produced Helium-3 combines to form the Helium nucleus an emit 2 protons
Steps 1 and 2 are take place two times (producing 26 MeV energy) before the step three (the combination of the formed helium-3) takes occurs.