Answer:
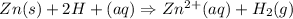
Step-by-step explanation:
Given that,
Anode : Zn electrode in a solution of 0.050 M Zn(NO₃)₂
Cathode : Pt electrode with 0.500 atm H₂(g) in 0.010 M HNO₃
Anode :
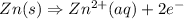
Cathode :
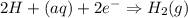
We need to write the overall balanced cell reaction
Using anode and cathode
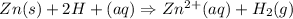
Hence, This is required answer.