Answer:
Two of the three salts are soluble.
One of the three salts is soluble.
Step-by-step explanation:
One or two of the three salts would be soluble in water depending on if the water is cold or hot.
According to the solubility rules of chloride salts, all chlorides are soluble in cold water except for those of silver (Ag), lead (Pb), and mercury (I) (Hg).
This thus means that both AgCl and
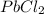 would be insoluble in cold water while LiCl would be soluble.
would be insoluble in cold water while LiCl would be soluble.
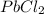 is, however soluble in hot water.
is, however soluble in hot water.
Hence, in cold water, one of the three salts (LiCl) would be soluble, while in hot water, two of the three salts (LiCl and
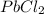 ) would be soluble.
) would be soluble.