Answer:
0.0002 M
Step-by-step explanation:
The molarity of the HCl required would be 0.0002 M.
First, let us consider the balanced equation of the reaction:
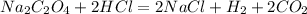
Stoichiometrically, 1 mole of
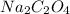 reacts with 2 moles of
reacts with 2 moles of
 for a complete neutralization reaction.
for a complete neutralization reaction.
Recall that: mole =

Mole of 0.550 g sodium oxalate = 0.550/134 = 0.0041 mole
If 1 mole
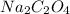 requires 2 moles HCl, then 0.0041 mole will require:
requires 2 moles HCl, then 0.0041 mole will require:
0.0041 x 2 = 0.0082 mole HCl
Volume of the HCl = 40.95 L
Molarity = mole/volume
Hence, molarity of the HCl = 0.0082/40.95 = 0.0002 M