Answer:
Since water has a higher specific heat than copper.
Step-by-step explanation:
Dimensionally speaking, the specific heat of a material (
 ) is represented by:
) is represented by:
![[c] = ([Energy])/([Mass]\cdot [Temperature])](https://img.qammunity.org/2021/formulas/physics/high-school/uxs890l2wpitlho0o4uk21gi44yh07gm7x.png)
The specific heats of water and copper are
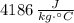 and
and
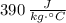 , respectively. Let suppose that temperature change and masses of water and copper are the same. Then, a kilogram of water takes a longer time than a kilogram of copper since the first has a higher specific heat.
, respectively. Let suppose that temperature change and masses of water and copper are the same. Then, a kilogram of water takes a longer time than a kilogram of copper since the first has a higher specific heat.