Answer:
pH=0.22.
![[OH^-]=1.66x10^(-14)M](https://img.qammunity.org/2021/formulas/chemistry/college/769t83ddumwqlcy1cyrl1jacal1fy9jwmd.png)
Step-by-step explanation:
Hello,
In this case, since the pH is directly computed from the given concentration of hydronium ions:
![pH=-log([H_3O^+])=-log(0.6M)=0.22](https://img.qammunity.org/2021/formulas/chemistry/college/c0kaoga7ev6parrcbv7chxfboq0wb1l9uv.png)
It is widely known that the pH and POH are directly related via:
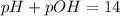
Therefore, the pOH is:
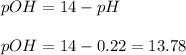
Thus, the concentration of hydroxyl ions are computed from the pOH:
![pOH=-log([OH^-]}\\\\](https://img.qammunity.org/2021/formulas/chemistry/college/iodxvw4fwsophl7l963v2zsdkjvl3qt4op.png)
![[OH^-]=10^{-pOH]=10^(-13.78)](https://img.qammunity.org/2021/formulas/chemistry/college/7ua54tfvhtqqbj5agonei6nwhtg7nne54v.png)
![[OH^-]=1.66x10^(-14)M](https://img.qammunity.org/2021/formulas/chemistry/college/769t83ddumwqlcy1cyrl1jacal1fy9jwmd.png)
Regards.