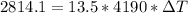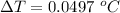Answer:
The maximum temperature increase is
Step-by-step explanation:
From the question we are told that
The mass of the bullet is
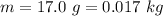
The speed is
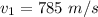
The mass of the water is
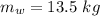
The velocity it emerged with is
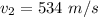
Generally due to the fact that energy can nether be created nor destroyed but transferred from one form to another then
the change in kinetic energy of the bullet = the heat gained by the water
So
The change in kinetic energy of the water is
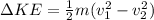
substituting values
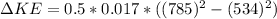
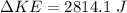
Now the heat gained by the water is

Here
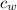 is the specific heat of water which has a value
is the specific heat of water which has a value
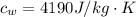
So since
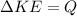
we have that