Answer:
Lime water,
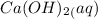 is formed.
is formed.
Step-by-step explanation:
Lime-water is a clear and colourless dilute solution of aqueous calcium hydroxide salt.
Small amounts of calcium hydroxide salt,
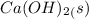 is sparsely soluble at room temperature when dispersed vigorously. if in excess, a white suspension called 'milk of lime'is formed.
is sparsely soluble at room temperature when dispersed vigorously. if in excess, a white suspension called 'milk of lime'is formed.
I hope this explanation is helpful.