Answer:
![[N_2]_(eq)=[H_2]_(eq)=0.09899M](https://img.qammunity.org/2021/formulas/chemistry/college/ogcpgt3ku2qzlko237jrpdo3dykwyw9d6a.png)
![[NO]_(eq)=0.00202M](https://img.qammunity.org/2021/formulas/chemistry/college/jgipv2bxdyfxnemmparkulxtemisd6id22.png)
Step-by-step explanation:
Hello,
In this case, for the given chemical reaction:
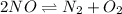
We know the equilibrium constant and equilibrium expression:
![Kc=2.4x10^3=([N_2][O_2])/([NO]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/v485v8epdb613asjm7ks0xys3fnplcq3mz.png)
That in terms of the reaction extent
 (ICE procedure) we can write:
(ICE procedure) we can write:
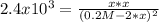
In such a way, solving for
 by using a quadratic equation or solver, we obtain:
by using a quadratic equation or solver, we obtain:
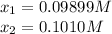
Clearly the solution is 0.09899M since the other value will result in a negative equilibrium concentration of NO. In such a way, the equilibrium concentrations of all the species are:
![[N_2]_(eq)=[H_2]_(eq)=x=0.09899M](https://img.qammunity.org/2021/formulas/chemistry/college/q446egpj2pckyizbbetkmsxu4qn60f8cl1.png)
![[NO]_(eq)=0.2M-2*0.09899M=0.00202M](https://img.qammunity.org/2021/formulas/chemistry/college/xzky325tk9auwqbxk10duzsfw1ngcaf8zn.png)
Regards.