Answer and Explanation:
a. The equation of K of this reaction is shown below:-
3 A + 5 B + 4 C↔5 D + 7 E + F
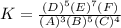
b. The moles of compound F is shown below:-
3 A + 5 B + 4 C↔5 D + 7 E + F
2 moles
Now, the mole of produced is
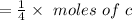
Now, we will the value of c by using the above equation
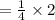
After solving the above equation we will get
0.5 moles