Answer:
The answer is "Option B"
Step-by-step explanation:
From the query, the following knowledge is derived:
Yield in percentage = 47%
Performance of theory = 4860 g
Actual yield Rate =?
The percentage return is defined simply by the ratio between both the real return as well as the conceptual return multiplied by the 100. It's also represented as numerically:
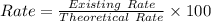
Now We can obtain the percent yield as followed using the above formula:
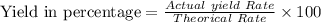
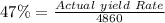
The value of the Actual yield Rate =

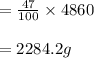
The Actual yield Rate= 2284.2 g.