Answer:
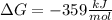
Step-by-step explanation:
Hello,
In this case, we must remember that the Gibbs free energy is defined in terms of the enthalpy, temperature and entropy as shown below:
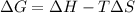
In such a way, for the given data, we obtain it, considering the conversion from J to kJ for the entropy in order to conserve the proper units:
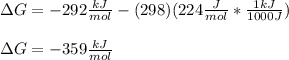
Best regards.