Answer:
14.07 units of joules is removed for each joules of electricity used
Step-by-step explanation:
The heated room temperature
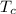 = 61 °F
= 61 °F
The outside temperature
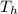 = 98 °F
= 98 °F
For conversion from fahrenheit to kelvin we use the equation
(32°F − 32) × 5/9 + 273.15
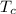 = 289.26
= 289.26
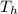 = 309.82
= 309.82
For air conditioning,
COP =
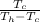
COP =
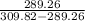 = 14.07
= 14.07
This means that 14.07 units of joules is removed for each joules of electricity used