Answer:
B. pOH = 14 - pH and D. pH = 14 - pOH.
Step-by-step explanation:
Hello,
In this case, we must remember that pH and pOH are referred to a measure of acidity and basicity respectively, since pH accounts for the concentration of H⁺ and pOH for the concentration of OH⁻ in a solution. In such a way, since the maximum scale is 14, we say that the addition between the pH and pOH must be 14:
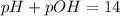
Therefore, the correct answers are B. pOH = 14 - pH and D. pH = 14 - pOH since the both of them are derived from the previous definition.
Best regards.