Answer:
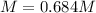
Step-by-step explanation:
Hello,
In this case, considering that the solution is formed by NaCl as the solute and water as the solvent, we can compute the molarity as shown below:
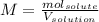
Whereas the volume of the solution must be in liters. In such a way, since the addition of sodium chloride does not significantly changes the volume of the solution we can say it remains in 100 mL (0.100 L) and the moles of sodium chloride are computed by using its molar mass (58.45 g/mol):
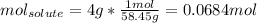
Therefore, the molarity is:
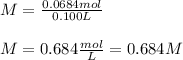
Regards.