Answer:
The freezing point will be -2.046°C.
Step-by-step explanation:
The freezing point depression equation is
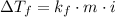
Where;
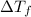 = The temperature depression of the freezing point
= The temperature depression of the freezing point
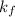 = The constant of freezing point depression which is solvent dependent = 1.86°C/m
= The constant of freezing point depression which is solvent dependent = 1.86°C/m
i = The number of particles the substance decomposes into in solution = 1 for sugar (a covalent compound)
m = The molality of the solution = 1.1
Therefore, we have;
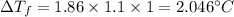
Therefore the freezing point will be 0 - 2.046°C = -2.046°C.