Answer:
The number of balloons is 948.8.
Step-by-step explanation:
The number of balloons can be calculated as follows:
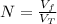
Where:
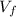 : is the volume at 1.086 atm
: is the volume at 1.086 atm
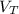 : is the balloon volume = 2.414 L
: is the balloon volume = 2.414 L
The volume at 1.086 atm can be found using Boyle's law:
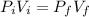
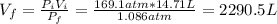
Now, the number of balloons is:
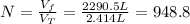
Therefore, the number of balloons is 948.8.
I hope it helps you!