Answer:
E = 1.24MeV
Step-by-step explanation:
The photon travels at the speed of light, 3.0 ×
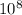 m/s, and given that its frequency = 1 picometer = 1.0 ×
m/s, and given that its frequency = 1 picometer = 1.0 ×
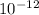 m.
m.
Its energy can be determined by;
E = hf
= (hc) ÷ λ
where E is the energy, h is the Planck's constant, 6.626 ×
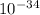 Js, c is the speed of the light and f is its frequency.
Js, c is the speed of the light and f is its frequency.
Therefore,
E = (6.626 ×
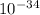 × 3.0 ×
× 3.0 ×
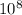 ) ÷ 1.0 ×
) ÷ 1.0 ×
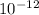
= 1.9878 ×
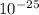 ÷ 1.0 ×
÷ 1.0 ×
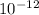
E = 1.9878 ×
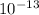 J
J
But, 1 eV = 1.6 ×
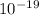 J. So that;
J. So that;
E =
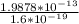
= 1242375 eV
∴ E = 1.24MeV
The energy of the photon is 1.24MeV.