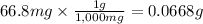Answer:
0.0668g
Step-by-step explanation:
Step 1: Given data
Concentration of lead: 668 ppm (mg/kg)
Mass of water: 100.0 g
Step 2: Convert the mass of water to kilograms
We will use the relationship 1 kg = 1,000 g.
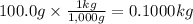
Step 3: Calculate the mass of lead in 0.1000 kg of water
There are 668 mg of Pb in 1 kg of water.
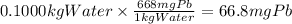
Step 4: Convert the mass of Pb to grams
We will use the relationship 1 g = 1,000 mg.