Answer:
The new volume will be 808 L
Step-by-step explanation:
Charles's law is a law that says that the volume of gas at constant pressure is directly proportional to its absolute temperature (in degrees Kelvin), that is, when the amount of gas and pressure are kept constant, the quotient between volume and temperature will always have the same value:
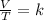
Having a certain volume of gas V1 that is at a temperature T1 at the beginning of the experiment, by varying the volume of gas to a new value V2, the temperature will change to T2 and the following will be fulfilled:
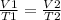
In this case:
- V1= 625 L
- T1= 273 K
- V2= ?
- T2= 353 K
Replacing:
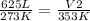
Solving:
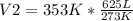
V2= 808 L
The new volume will be 808 L