Answer:
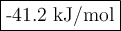
Step-by-step explanation:
Balanced equation: CO(g) + H₂O(g) ⟶ CO₂(g) + H₂(g)
We can calculate the enthalpy change of a reaction by using the enthalpies of formation of reactants and products
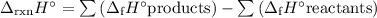
(a) Enthalpies of formation of reactants and products
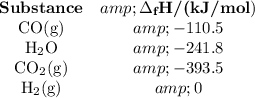
(b) Total enthalpies of reactants and products
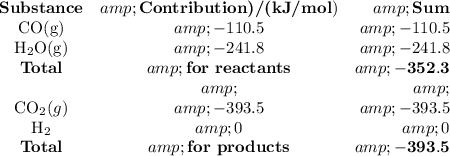
(c) Enthalpy of reaction
