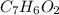Answer:
The molecular formula of the compound is
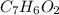 .
.
Step-by-step explanation:
Let consider that given percentages are mass percentages, so that mass of each element are determined by multiplying molar massof the organic acid by respective proportion. That is:
Carbon
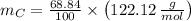
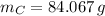
Hydrogen
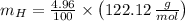
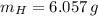
Oxygen
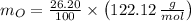
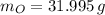
Now, the number of moles (
 ), measured in moles, of each element are calculated by the following expression:
), measured in moles, of each element are calculated by the following expression:
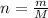
Where:
 - Mass of the element, measured in grams.
- Mass of the element, measured in grams.
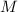 - Molar mass of the element, measured in grams per mol.
- Molar mass of the element, measured in grams per mol.
Carbon (
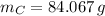 ,
,
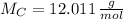 )
)
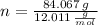
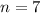
Hydrogen (
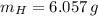 ,
,
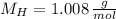 )
)
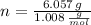
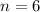
Oxygen (
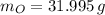 ,
,
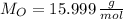 )
)
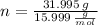
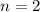
For each mole of organic acid, there are 7 moles of carbon, 6 moles of hydrogen and 2 moles of oxygen. Hence, the molecular formula of the compound is: