Answer:
The value of Kp at this temperature is 9.0*10³
Step-by-step explanation:
The equilibrium constant Kp describes the relationship that exists between the partial pressures of the reactants and products, while Kc represents the relationship that exists between the concentrations of the reactants and products that participate in the reaction.
The general relationship between the constants Kp and Kc results:
Kp=Kc*
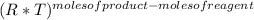
In this case:
- Kc= 2.2*10⁵
- R = gas constant = 0.0821
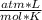
- T = Kelvin temperature = 298 K
- moles of gaseous products - moles of gaseous reactants = 1 - 2 = -1
Replacing:
Kp=2.2*10⁵*
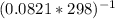
Solving:
Kp≅9.0*10³
The value of Kp at this temperature is 9.0*10³