Answer:
8.13 g/mol.
Step-by-step explanation:
The following formula gives us the relationship between the effusion rates of two gases and their molar masses:
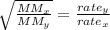
where x and y are respective sample gases and MM and rate are molar mass and rate of effusion respectively.
⇒
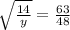
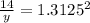
y= 14 /
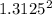 = 8.13 g/mol.
= 8.13 g/mol.