Answer:
The enthalpy of formation of CaF₂ is -1224.4 kJ.
Step-by-step explanation:
The enthalpy of formation of CaF₂ can be calculated as follows:
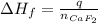
Where:
q: is the heat liberated in the reaction = -251 kJ
The number of moles of CaF₂ is:
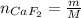
Where:
m: is the mass of CaF₂ = 16 g
M: is the molar mass of CaF₂ = 78.07 g/mol
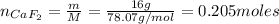
Now, the enthalpy of formation of CaF₂ is:
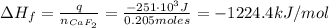
Therefore, the enthalpy of formation of CaF₂ is -1224.4 kJ.
I hope it helps you!